Answer:
25.4 L
Explanation:
Charles's Law

where:
- V₁ = first volume.
- T₁ = first temperature.
- V₂ = second volume.
- T₂ = second temperature.
Given:
- V₁ = 25 L
- T₁ = 25.0 °C
- T₂ = 30.0 °C
Convert Celsius to kelvin.
Kelvin = Celsius + 273.15
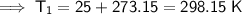
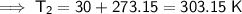
Substitute the given values into the formula:
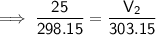
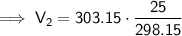
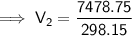
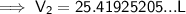
Therefore, the volume of air in the tire if the temperature increases to 30.0 °C is 25.4 L (nearest tenth).