Answer:
252.459 KJ/mol & no, that is not sufficient to break H-H bond.
Step-by-step explanation:
Calculate wavelength from the equation
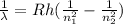 where n1 = 2 n2 = 5 Rh = 1.097X10^7 m^(-1)
where n1 = 2 n2 = 5 Rh = 1.097X10^7 m^(-1)
λ = 4.34X10^-7 m
Now calculate E using
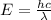 where E = Energy h = Plank's constant c = velocity of light (3X10^8 m/s)
where E = Energy h = Plank's constant c = velocity of light (3X10^8 m/s)
E = 4.194X10^-19 J
now to convert Joules to Joule/mol we need to multiply the energy by avogadro's number which is 6.023X10^23
E = 252458.9873 J/mol
Dividing this amount by 1000 would yield energy in KJ/mol
E = 252.46 KJ/mol
252.46<436 KJ/mol
Thus H-H bond can't be broken by this energy