Answer:

Step-by-step explanation:
Given Data:
Isotope A mass = 28.776 g
Isotope A abundance = 15.7%
Isotope B mass = 25.992 g
Isotope B abundance = 1.3%
Isotope C mass = 27.004 g
Isotope C abundance = 83%
Required:
Average Atomic Mass = ?
Formula:
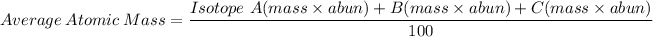
Solution:
![\displaystyle Average \ Atomic \ Mass = (28.776* 15.7 +25.992 * 1.3+27.004 * 83 )/(100) \\\\Average \ Atomic \ Mass=(451.7832 + 33.7896+2241.32)/(100) \\\\Average \ Atomic \ Mass=(2726.9)/(100) \\\\Average \ Atomic \ Mass=27.269 \ amu\\\\\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/chemistry/college/lb6zvl8w77yq90nvoo8tpq1cuxin9gnzmr.png)