Answer:
1. x=1.25; y=1.75
2. x=15; y=4
3. x=3; y=2
Explanation:
Because of the parallel lines, you're working with similar triangles. Therefore, the side lengths of the smaller triangle (in each figure) are proportional to those of the larger. Set up a proportion for x and solve; then for y.
Ex:
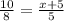 ⇒ 50=8(x+5)=8x+40 ⇒ 10=8x ⇒ x=1.25
⇒ 50=8(x+5)=8x+40 ⇒ 10=8x ⇒ x=1.25
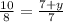 ⇒ 35=28+4y ⇒ 7=4y ⇒ y=1.75
⇒ 35=28+4y ⇒ 7=4y ⇒ y=1.75
NOTE: Be sure to set up the correct corresponding sides!