Answer:
ΔT = 200ºC to ΔT = 11ºC
Step-by-step explanation:
This is a thermal expansion exercise, for a material with volume the expression
ΔV = β V₀ ΔT
for when the changes are small
β = 3 aα
in the exercise they indicate the coefficient of thermal expansion of the liquid and solid
α_solid = 5 10⁻⁵ ºC⁻¹
α_liquid = 3 to 55 10⁻⁴ ºC⁻¹ = 30 to 550 10⁻⁵ ºC⁻¹
it is requested to increase the temperature so that the height of the liquid rises 5% = 0.05 inside the container
The expression for the volume of a body is its area for the height
V₀ = π r² h
the final volume when heated changes as the radius and height change, suppose the radius is equal to the height
r = h
V_f = π (h + 0.05h)² (h + 0.05)
V_f = pi h² 1,05³
V_f = 1.157525 V₀
the volume variation is
V_f - V₀ = 1.15 V₀ - V₀
ΔV = 0.15 V₀
therefore, so that the total change in the volume of liquid and solid is the desired
ΔV = ΔV_{liquid} - ΔV_{solid}
let's write the dilation equations for the two elements
ΔV_{liquid} = 3 α_{liquid} V₀ ΔT
ΔV_{solid} = 3 α_{solid} V₀ ΔT
we are assuming that the volumes of the solid and liquid are initial, as well as their temperatures
we substitute
0.15 V₀ = 3 α_{liquid} V₀ ΔT - 3 α_{solid} V₀ ΔT
0.15 = 3 ΔT ( α_{liquid} - α_{solid})
ΔT =
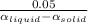
let's calculate for the extreme values of the liquid
α_{liquid} = 30 10⁻⁵ ºC⁻¹
ΔT =
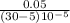
ΔT = 5000/25
ΔT = 200ºC
α_{liquid} = 5.5 10-5
ΔT = \frac{0.05}{( 550 -5 ) 10^{-5}}
ΔT = 5000 / 450
ΔT = 11ºC