Answer:
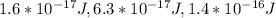
Step-by-step explanation:
The equation for the energy of a particle in a 1d "box" (line) is
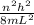
n equals any positive integer, and h is a constant. m is the mass of the electron, and l is the length of the box.
when n = 1, plug in 6.626*10^-34 for h, 9.1093837 *10^-31 for m, and 6.2*10^-11 m for L
(6.626*10^-34)^2 / (8* 9.1093837*10^-31 * (6.2*10^-11)^2 ) which equals
1.56725802e-17 joules
when n = 2, the energy is greater than when n = 1 by a factor of 2^2, or 4. so, the energy it has when n = 2 is
1.56725802e-17 * 4, or 6.26903208e-17 joules
when n = 3, the energy equals
3^3 * 1.56725802e-17, or 9 * 1.56725802e-17, or 1.41053222e-16 joules
round to 2 sig figs to get
1.6 * 10^-17 J, 6.3 * 10^-17 J, and 1.4 * 10^-16 J