Answer: a) pH = 2.56 , acidic
b) pH = 2.38, acidic
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/79qpm3uwz8hayr1qtyvcw8.png)
a)
![[H^+]=2.7* 10^(-3)](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/k1agtnngluidxx4g086xy2.png)
Putting in the values:
![pH=-\log[2.7* 10^(-3)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/mv3hpxi9oqcmlu7xup21ho.png)
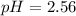
Thus as pH is less than 7, the solution is acidic.
b)
![[H^+]=4.1* 10^(-3)](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/fpzaqyowq34tg6ndeveiy5.png)
Putting in the values:
![pH=-\log[4.1* 10^(-3)]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/b0qutdvh9phsxjz4sftuol.png)
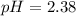
Thus as pH is less than 7, the solution is acidic.