Answer:
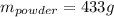
Step-by-step explanation:
Hello there!
In this case, according to the composition of the black powder - 75% potassium nitrate, 15% charcoal and 10% sulfur, we can see that starting by 65.0 grams of charcoal, the produced amount of the black powder would be:

Best regards!