Answer:
3.02 atm
Step-by-step explanation:
To find the final pressure, you need to use the Combined Gas Law:
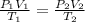
In this equation, "P₁", "V₁", and "T₁" represent the initial pressure, volume, and temperature. "P₂", "V₂", and "T₂" represent the final pressure, volume, and temperature. You first need to convert the temperatures from Celsius to Kelvin (1°C = 273 K). You should convert the initial pressure from kPa to atm (1 atm = 101.3 kPa).
P₁ = 536 kPa / 101.3kPa = 5.29 atm P₂ = ? atm
V₁ = 2.50 L V₂ = 3.75 L
T₁ = 75.0 °C + 273 = 348 K T₂ = 25.0°C + 273 = 298 K
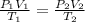 <----- Combined Gas Law
<----- Combined Gas Law
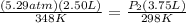 <----- Insert values
<----- Insert values
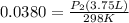 <----- Simplify left side
<----- Simplify left side
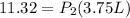 <----- Multiply both sides by 298 K
<----- Multiply both sides by 298 K
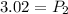 <----- Divide both sides by 3.75 L
<----- Divide both sides by 3.75 L