Answer:
pH = 7.08
Step-by-step explanation:
We can solve this problem using Henderson-Hasselbach's equation:
- pH = pKa + log
![([ClO^-])/([HClO])](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/hw0s53ngq6bwzqbrxjbq1y.png)
With the given concentrations and volumes, we can calculate the new values for [ClO⁻] and [HClO] when the solutions are mixed:
- Total volume = 550.0 mL + 275.0 mL = 825.0 mL
- [HClO] = 550 mL * 0.30 M / 825 mL = 0.20 M
- [ClO⁻] = 275 mL * 0.20 M / 825 mL = 0.07 M
Now we calculate pKa:
Finally we calculate the pH:
- pH = 7.54 + log
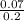 = 7.08
= 7.08