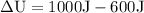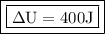Hello..!
The first law of thermodynamics relates work and transferred heat exchanged in a system through a new thermodynamic variable, internal energy. This energy is neither created nor destroyed, only transformed.
We can think of gas as a thermodynamic system, all because gases can work and absorb heat, and then they can turn all that into energy.
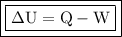
The formula for the change in energy is given by the first law of thermodynamics expressed as:
Being:
- ΔU = change in energy
- Q = added heat
- W = Work done
Problem:
A gas receives from an external thermal source an amount of heat equal to 1000 J. This energy, in addition to producing heating in the gas, causes its expansion, with consequent performance of work equivalent to 600 J. What was the change in the internal energy of the gas? gas?
Data:
- ΔU = ¿? (Meet)
- Q = 1000 J
- W = 600 J
Now adding the data in the formula to find the energy change: