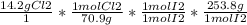Answer:
50.8g of iodine is produced.
Step-by-step explanation:
You have to do stoichiometry. To get grams of iodine produced.
- Start with grams of chlorine that you're given
- Convert these to moles (using molar mass)
- Convert moles of Cl2 into moles of I2
- Convert moles of I2 into grams of I2 (using molar mass)
(Remember to multiply across the top and divide by the bottom)