Answer:
density = 1.43 × 10⁻³ g/cm³
Explanation:
We are told that oxygen gas of mass 2.23 kg has a volume of 1.56 × 10⁶ cm³, and we are told to find its density in g/cm³.
To do this we have to first convert the mass, which is given in kg, to g:
mass = 2.23 kg = 2.23 kg × 1000
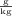
= 2230 g
Next, we can find the density of the gas using the following formula:
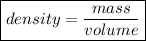
⇒ density =
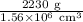
= 1.43 × 10⁻³ g/cm³
Therefore, the density of the oxygen gas is 1.43 × 10⁻³ g/cm³.