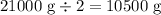Answer:
10,500 g
Explanation:
Half-life is the length of time it takes for half of the radioactive atoms of a radionuclide to decay. In this scenario, the amount of radioactive atoms is halved after 25 minutes. After 75 minutes, then, the amount of radioactive atoms is halved three times. Therefore:
1st half-life:
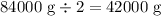
2nd half-life:
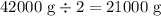
3rd half-life: