The relationship between wavelength and frequency is given by
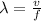
Where
λ = wavelength
v = speed of light
f = frequency
The speed of light in free space is 2.998×10⁸ m/s
For the given case, the wavelength is 442×10⁻⁹ m
So, the corresponding frequency of this color of light is
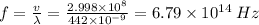
Therefore, the frequency of this color of light is 6.79×10¹⁴ Hz
Option A is the correct answer.