Assuming the number of moles give is of CO₂, since we know that 1 mol is equivalent to 22.4 L in this case, we can use rule of three to answer this:
V ---- n
x ---- 3.75 mol
22.4 L ---- 1 mol
So, we have the follwing relation:
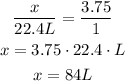
So, we need a volume of 84 L.