Answer:
0.17M. Option B is correct
Explanations:
The formula calculating the molarity of a solution is given as:
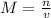
where:
n is the moles of sodium chloride
v is the volume of the solution
Given the following parameters;
The volume of the solution = 3.0L
Determine the moles of NaCl
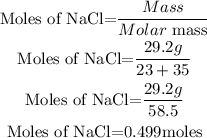
Calculate the required molarity
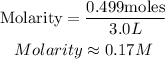
Hence the molarity of the solution is 0.17M