We are required to calculate the number of moles.
Given:
concentration = 11.7%
molar mass = 118.5 g/mol
mass = 2.780 kg = 2780 g
To calculate the number of moles we can use the following equation:
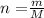
Where m is the mass and M is the molar mass of the unknown solution.
n = 2780 g /118.5 g/mol
n = 23.46 moles