Given:
The initial quantity of the actinium that will decay is 134720 grams.
Time, t=50 days
The half-life of the decaying quantity is 10 days.
To find the quantity that still remains after time t:
The half-life formula is given by,
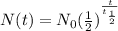
On substitution we get,
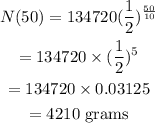
Hence, the quantity that still remains after time 50 days is 4210 grams.