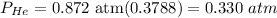Answer
Procedure
The total pressure of a mixture of gases can be defined as the sum of the pressures of each individual gas:

The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas.
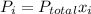
The molar fraction of He is determined by dividng the moles of He over the total moles
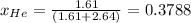
Finally, the total pressure of He is