Formula that contains K+ and PO4-3
To find this formula, we need to exchange the oxidation states:
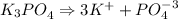
The charge of the compound has to be 0.
---------------------------------------------------------
Let's see:
K+ is a cation.
PO4-3 is an anion.
These ions are from this compound K3PO4, a salt.
When a salt ionizes, it gives anion and cation.
Anion and cation are ions.