Step 1
Boyle's law states that the volume of a certain mass of a gas, at a constant temperature, varies inversely with the pressure exerted on it.
Mathematically:
P1 x V1 = P2 x V2 (1)
Where,
P1 = initial pressure
V1 = initial volume
----
P2 = final pressure
V2 = final volume
--------------------
Step 2
Information provided:
P1 = 5.0 atm
V1 = 40 L
----
P2 = 1.0 atm
V2 = unknown
---------------------
Step 3
V2 is found from (1) as follows:
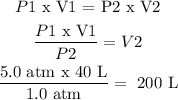
Answer: b. 200 L