Since 15.3 L was used, we can convert this to mass using the density:
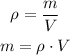
Using the density of 0.755 g/mL, we need to convert the volume to mL, so:
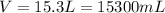
Now, using the volume and the density, we have:
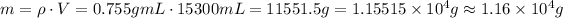
So, the mass in grams is approximately 1.16 x 10⁴ g.
To convert to ounces, we can divide the value in grams by approximately 28.349523:
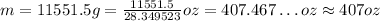
So, the mass in ounces is approximately 407 oz.