Given data:
The standard expression for the proportionality is,
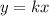
It means the graph must pass through the origin.
5)
Thus, the amount of money raised by Phil's is proportionally related.
6)
The unit rate change is the slope of the line which is,
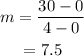
Thus, the unit rate change is $7.5/mile.