To balance the equation we can start with oxygen. We have three oxygen atoms in the reactants and two in the products, to balance, we put the opposite coefficient to the number of oxygen atoms. That is to say that in the reactants we place the coefficient 2 and in the products the coefficient 3.
To complete the balance we put the coefficient two on the KCl molecule to balance the chlorine and potassium. So, the balanced equation will be:
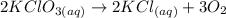
Now, we find the moles present in 13 grams of oxygen. We divide the grams of oxygen by the molar mass of oxygen which is equal to 31.998 g/mol. The moles of oxygen will be:
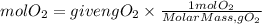
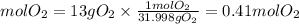
By stoichiometry, we find the moles of potassium chlorate. We have that the ratio KClO3 to O2 is 2/3. So, the moles of KClO3 needed will be:
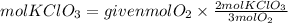
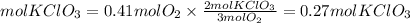
To produce 13 grams of oxygen are needed 0.27 moles of KClO3
Answer: Last option. 0.27 mol