Answer:
0.0284moles
Explanations:
The formula for calculating the moles of an element is given as;
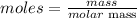
Determine the mass and molar mass of copper
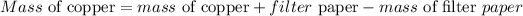
Given the following parameters
Mass of copper+filter paper = 2.4581grams
Mass of filter paper = 0.6503grams
Substitute
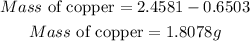
Determine the moles of copper
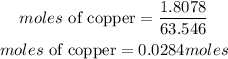
Hence the required moles of copper is 0.0284mol