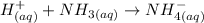The net ionic equation between HClO4 + NH3:
General steps to writing net ionic equation:
1. Write a balance molecular formula
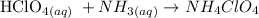
2. write the state of each substance (l, g, aq or s)
3. Split strong electrolytes to ions
So let us plit from the above equation:
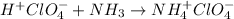
4. Cross out the spectator ions on both sides, in this case cross out ClO4^-
5. The remainingsubstances are the net ionic equation: