1) List known values
Initial concentration (C1): 3.49 ppm
Initial volume (V1): 200.00 mL
Final concentration (C2): 22.0 ppm
Final volume (V2): ?
ppm = mg/L
2) Set the equation
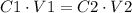
3) Change the units of volume
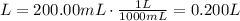
4) Plug in known values and solve for V2

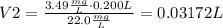
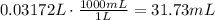
If we want to make 200.00 mL of 3.49 ppm solution, we have to measure 31.73 mL of 22.0 ppm solution.