Answer: Initial volume = 18 L
Calculations:
We are given -
• Volume 1 (V1) = ?
,
• Temperature (T1) = 120K
,
• Volume 2(V2) = 45L
,
• Temperature (T2) = 300K
We can use Charles law equation to solve for initial volume :
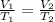
Replacing the given parameters into Charles law equation, we get that V1 is :
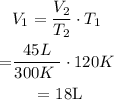
This means that the initial volume = 18 L