Chemistry => Chemical Reactions => Balancing Equations
We have a decomposition reaction, a compound breaks its bond to form two.
To balance the equation we must start by counting the atoms of each element on both sides of the reaction.
The element Hg is already balanced, we have 1 atom on each side. We will balance oxygen atoms. We have 2 oxygen atoms in the products, so we write coefficient 2 in the HgO molecule.
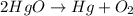
Now, the number of Hg atoms has changed on the reactants. We have 2 Hg atoms on the reactants so we put coefficient 2 on the Hg molecule. We will have:
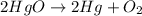
Now, the equation is balanced.
Answer: 2HgO → 2Hg+O2