Answer: for the given aqueous solution, the [OH-] value is 1.0 x 10^-11 M
Step-by-step explanation:
The question requires us to calculate the concentration of OH- ions ([OH-]) in an aqueous solution, knowing that the concentration of H+ ions ([H+]) is 1.0 x 10^-3 M.
To solve this problem, we can consider the self-ionization of water and its correspondent ionization constant:
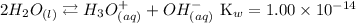
Note that the constant of equilibrium expression for the reaction above, Kw, can be written as:
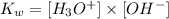
Also, note that H3O+ ions are equivalent to H+ ions.
Therefore, we can rearrange the equation above to calculate the concentration of OH- ions in an aqueous solution, knowing that the equilibrium constant for the self ionization of water is 1.00 x 10^-14 and that the concentration of H+ ions in the solution is 1.0 x 10^-3:
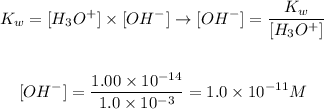
Therefore, for the given aqueous solution, the [OH-] value is 1.0 x 10^-11 M.