We are given that a hot iron block is placed inside room temperature water. This means that there is a heat transfer from the iron block into the water. The formula for the heat that is transferred is given by:
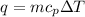
Where "m" is the mass, "cp" is the specific heat and "delta T" is the difference in temperature.
Since the heat leaves the iron and goes into the water, by the principle of conservation of energy, we have the following relationship:
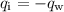
Replacing the formulas for the heat:
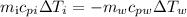
Now we establish the difference in temperature:
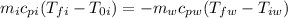
Now we solve for the specific heat of iron:
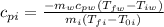
Replacing the values:
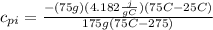
Solving the operations:
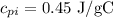
Therefore, the specific heat of iron is 0.45 J/g°C