Step 1 - Understanding what happens during a chemical reaction
During a chemical reaction, substances are transformed. The susbtances already present before the reaction are called reactants, whereas the susbtances formed by the reaction are called products:
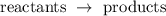
A chemical reaction is a little bit like Lego playing: no new atom is created nor destroyed, they are just rearranged. This rearrangement is possible because the reactants' chemical bonds are broken, and new bonds are formed.
Step 2 - Finding the correct alternative
As we have seen in step 1, no new atoms are formed. The atoms are the same, they are just joined together in a different way.
Therefore, item a) is not correct.