The required volume of NaF is 765.97 mL.
0.18 moles of SrF2 are formed.
Volume calculation
- First, we need to know the molar mass of the reactants:
SrCl2 molar mass = 158.53 g/mol
NaF molar mass = 41.98 g/mol
- Second, we need to know the amount of moles that are in the 573mL of SrCl2:
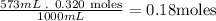
So, there are 0.18 moles of SrCl2 in the 573mL of a 0.320 M solution of SrCl2.
- Now, we relate the stoichiometry of the equation to the number of moles of SrCl2 that we have:
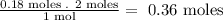
So, we need 0.36 moles of NaF.
- Finally, we calculate the volume of 0.470M NaF that is needed to react completely with the SrCl2:
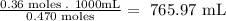
So, the required volume of NaF is 765.97 mL.
Moles of SrF2 formed
From the balanced equation, we know that 1 mole of SrF2 is formed from 1 mole of SrCl2, so we calculate the moles of SrF2 formes from the 0.18 moles of the SrCl2 solution:
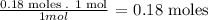
As the relation is 1 to 1, 0.18 moles of SrF2 are formed.