We know that the product of pressure and the volume divided by the temperature is a constant if the mass of the gas remain constant, this means that:
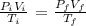
First we need to convert the temperature to the absolute scale:
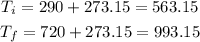
The final volume is 2.5Vi. Plugging the values given and solving for the final pressure we have that:
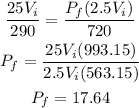
Therefore the final pressure is 17.64 psi.