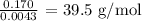Answer:
Step-by-step explanation:
Here, we want to get the molar mass of the gas
We start by getting the number of moles of the gas used in the experiment
We can get this bu using the ideal gas equation as follows;

Before we use these values, we have to convert Pressure to atm (We can do this by dividing the pressure value by 760
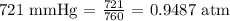
Substituting the values, we have:
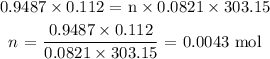
To get the molar mass of the gas, we have to divide the mass by the number of moles of the gas used in the experiment
Mathematically, we have that as: