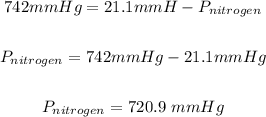Answer
720.9 mmHg
Step-by-step explanation
Gas collected over water must always account for the saturated vapor pressure.
Note that the saturated vapor pressure of water at 23 °C = 21.1 mm Hg.
Therefore atmospheric pressure is
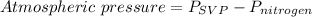
The atmospheric pressure given = 742 mmHg, Psvp = 21.1 mmHg, thus, we