The first step to solve this problem is to find how many molecules of scheelite contain 1 billion atoms. To do it, divide 1 billion by 4, this is because each molecule of scheelite contains 4 atoms:
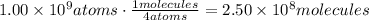
Now, use Avogadro's number to find the amount of moles in 2.50x10^8 molecules:
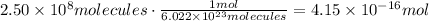
Use the molecular weight of scheelite to convert the obtained moles to grams:
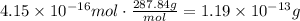
The answer is 1.19x10^-13 grams.