Answer:
2.38 g of oxygen (O2).
Step-by-step explanation:
What is given?
Mass of potassium chlorate (KClO3) = 6.06 g.
Molar mass of KClO3 = 122.4 g/mol.
Molar mass of oxygen (O2) = 32 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation. Remember that the decomposition of a compound produces two or more products:
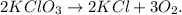
Now, let's convert 6.06 g of KClO3 to moles using its molar mass:

You can see in the chemical equation that 2 moles of KClO3 produce 3 moles of O2. By doing a rule of three with this data, we obtain that:
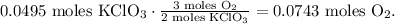
The final step is to convert from 0.0743 moles of O2 to grams using its molar mass, like this:
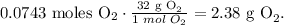
The answer is that we will produce 2.38 g of oxygen (O2) from the decomposition of 6.06 g of potassium chlorate (KClO3).