The molecular formula of ammonium oxide is:
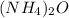
The molecular weight of it is 52.07 g/mol.
In one molecule of ammonium oxide there are two molecules of nitrogen, the molecular weight of it is 14 g/mol.
Let's use one mole of ammonium oxide for our calculations. In one mole of this substance there are 52.07g of the compound and 28 g of nitrogen (since there are two moles of it in ammonium oxide). Then, the composition would be:
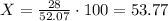
The percent compostion is 53.77%.