The question requires us to calculate the percent composition of carbon (C) in Fluticasone, given that its molecular formular is:
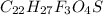
The percent composition corresponds to the percent by mass of each element in a compound. We can calculate it for a given element by dividing the amount, in mass of this element, contained in the molecule by the total mass of the molecule.
To solve this question, we need to calculate:
1) how much of carbon is there in the molecule given (considering its atomic mass and number of C atoms in the molecule)
2) the molar mass of the compound, considering all elements that compose it
3) the percent composition of C.
Next, we'll go through the steps above to solve the problem:
1) The atomic mass of carbon is 12.0107 u and we can see that there are 22 atoms of carbon in the molecular formula of Fluticasone. Thus, we can calculate the total amount of carbon in Fluticasone as:

Therefore, there are 264.235g of C in the molecule of Fluticasone.
2) Now, we need to calculate the total molar mass of Fluticasone, considering all elements that are part of this molecule. We'll need the following atomic masses:
atomic mass (C) = 12.107 u
atomic mass (H) = 1.00784 u
atomic mass (F) = 18.9984 u
atomic mass (O) = 15.999 u
atomic mass (S) = 32.065 u
We can calculate the molar mass of Fluticasone from the number of atoms of each element and their respective atomic mass:
![\begin{gathered} \text{molar mass of }Fluticasone\text{ = (22}*12.107)+(27*1.00784)+(3*18.9984)+(4*15.999)+(1*32.065) \\ \text{molar mass of Fluticasone = }446.62g/\text{mol} \end{gathered}]()
Therefore, the molar mass of Fluticasone is 446.62 g/mol.
3) Next, we calculate the percent composition of carbon in the molecule of Fluticasone by dividing the total amount of carbon in the molecule (264.235g of C) by the total mass of the molecule (446.62g of fluticasone), and then multiplying this value by 100%:
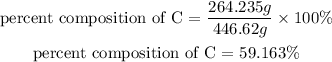
Therefore, there is 59.163% of C in the molecule of fluticasone.