Answer:
280000J
Step-by-step explanation:
The heat required can be calculated using the following equation
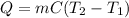
Where m is the mass, so m = 7 kg, C is the specific heat capacity, so C = 4000 J/Kg °C, and T2 and T1 are the temperatures, so T1= 7 °C and T2 = 17°C.
Therefore, by replacing the values, we get:
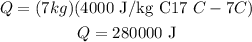
Then, the answer is 280000J