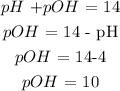Answer:
Step-by-step explanation:
We start by writing the dissociation equation to yield hydrogen and hydroxide ion
We have that as:
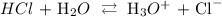
We have been told that the dissociation is 100% complete.
What this means is that the concentration of the acid is the concentration of the hydrogen ion
Thus, we have it that:
a)
![[H^+]\text{ = 0.00010 M}](https://img.qammunity.org/2023/formulas/chemistry/college/568s4p4zw1ny4nn0ob61wy98au6j6f9zii.png)
B) Mathematically:
![[H^+][OH^-]\text{ = 10}^(-14)](https://img.qammunity.org/2023/formulas/chemistry/college/kln7qy4dogaywr18id009e9gcmlxiddyyc.png)
We proceed to substitute for the concentration of the hydrogen ion:
![\begin{gathered} 0.0001\text{ }*\text{ \lbrack OH}^-]\text{ = 10}^(-14) \\ \\ [OH^-]\text{ = }(10^(-14))/(0.0001) \\ \\ [OH^-]\text{ = 1 }*\text{ 10}^(-10)\text{ M} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/ufsza5fnjcyq0q8obx7h77irtsb3hfynwe.png)
c) Mathematically:
![\begin{gathered} pH\text{ = -log\lbrack H}^+] \\ pH\text{ = -log\lparen0.0001\rparen} \\ pH\text{ = 4} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/xsufosuy9tr8ik77q1xaqyl3ugd2ypgxbb.png)
d) Mathematically: