Explanations:
The formula for calculating the number of moles of a compound is expressed as:
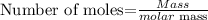
Given that mass of NaHCO3 = 1.0g
The molar mass of NaHCO3 = (1*23) + 1 + 12 + (3*16)
The molar mass of NaHCO3 = 23 + 13 + 48
The molar mass of NaHCO3 = 84g/mol
Get the moles of NaHCO3
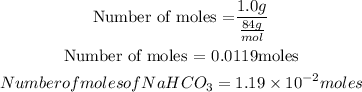
Get the moles of C2H3O2H (acetic acid)
Given the following parameters:
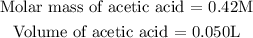

The limiting reagent will be the compound with the least number of moles. From the resulting moles, the compound that has the lower number of moles is acetic acid, hence the limiting reagent will be C2H3O2H
The excess reagent will be NaHCO3