The half life of a nuclear element tells us the time it takes for a radioative material to reduce to half its value.
If the Initial amount is A_0,
then after 1 half life, remaining = 1/2 A_0
then after 2 half life, remaining = (1/2) 1/2 A_0 = 1/4 A_0
...
The half life equation is
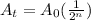
A_0 is initial amount
n is the number of half lives that pass in interval "t"
A_t is the undecayed amount after time interval "t"
a)
Given half life is 138 days and
138 * 6 = 828 days
So,
6 half lives occur in 828 days
b)
The amount of Polonium in the sample is >>>>
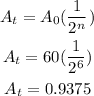
Rounded, that 0.94 grams
Correct Answer
A