The ideal gas law is given by:
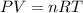
Where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant and T is the temperature.
In this formula, the temperature must be in Kelvin, that is, "K".
Therefore the correct option is a.