ANSWER
The number of moles of argon is 0.500 mole
Step-by-step explanation
Given data
The volume of argon at STP = 11.2L
Let x represents the number of moles of argon
Recall that, at STP, 1 mole is equivalent to 22.4L
Hence, we can calculate the number of moles of argon by using the conversion at STP
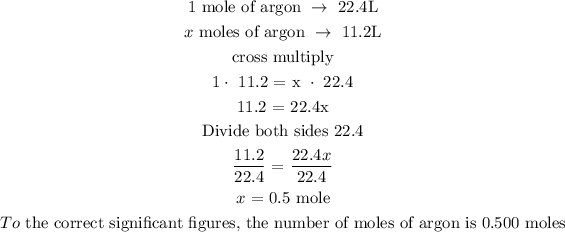
Therefore, the number of moles of argon to the correct significant figures is 0.500 mole