Answer:
Mass of MgO = 28.35grams
Mass of Sulphur = 11.29 grams
Explanations:
The balanced chemical equation between magnesium metal and sulphur dioxide is given as:
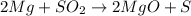
Determine the moles of magnesium
Mole = mass/molar mass
Mole of Mg = 17.1/24.305
mole of Mg = 0.704moles
According to stoichiometry, 2 moles of Mg produces 2 moles of MgO, hence the required mass of MgO will be:
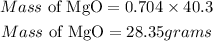
Similarly, 2moles of Mg produces 1 mole of sulphur, hence the mass of sulphur produced is;
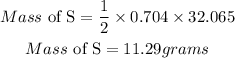
Hence the mass of magnesium oxide and the mass of sulphur that forms is 28.35grams and 11.29grams