Answer:
The volume of the objetc is 3.2 mL.
Step-by-step explanation:
Information given from the exercise:
- object mass: 9.6g
- initial volume: 10.0 mL
- final volume: 13.2 mL
The difference between theinitial volume and the final volume is the volume occupied by the 9.6g object:
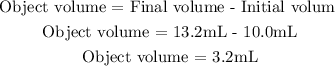
So, the volume of the objetc is 3.2 mL.