We know that two barometers are measuring the same pressure when the mass in their columns are equal, that is, the two barometers will measure the same when:
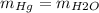
To find the mass of the barometer we need to remember that the mass is related to the density of the substance and its volume by:
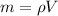
Hence the first equation takes the form:
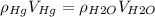
Now, let's assume the barometers are cylindrical, then their volume is given by:
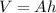
where A is the area of the base; let's further assume the area of the base is equal for both barometers; plugging this in our equation we have:
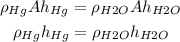
Hence we have the following equation relating the densities of the substances in the barometer and the height of the column in it:
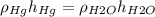
Now, before we plug the values of the densities we will convert the height of the mercury column to cm (this will make the operations easier), let's do that:
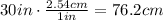
The density of mercury is 13.6 g/cm^3, the density of water is 1 g/cm^3; plugging the values we know we have that:
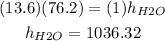
Hence, the height of the column of water is 1036.32 cm, let's convert this into inches:
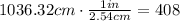
Therefore, the height of the column of water is 408 in tall.